Page 8 - BASIC CONCEPTUAL OF THERMOFLUID
P. 8
CHAPTER 1: CONCEPTUAL PRINCIPLE IN THERMOFLUIDS
1.4 Principles of a System, Boundary and Surrounding
These are some common definitions associated with the basic thermodynamics. every
thermodynamic study and analysis are related to these terms. let's get a quick review of these
terms. Thermodynamic system is the place which contains a certain quantity of matter in which
thermodynamic processes happen and thermodynamic analysis can be carried out. in the system,
the matter will consist of certain properties which can be altered by different processes like
transfer of mass and energy. Boundary is something which separates the system and the
surroundings. the boundary can be real or imaginary. sometimes a relative boundary is
considered so the boundary can be at rest or in a motion. Surrounding is everything external to
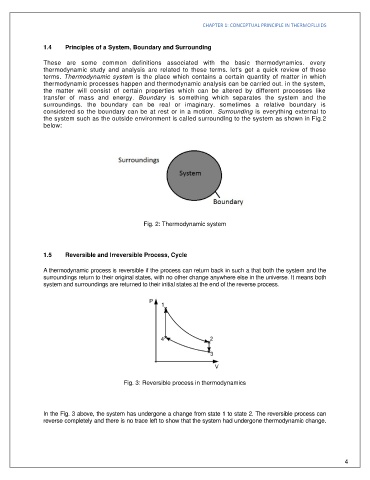
the system such as the outside environment is called surrounding to the system as shown in Fig.2
below:
Fig. 2: Thermodynamic system
1.5 Reversible and Irreversible Process, Cycle
A thermodynamic process is reversible if the process can return back in such a that both the system and the
surroundings return to their original states, with no other change anywhere else in the universe. It means both
system and surroundings are returned to their initial states at the end of the reverse process.
Fig. 3: Reversible process in thermodynamics
In the Fig. 3 above, the system has undergone a change from state 1 to state 2. The reversible process can
reverse completely and there is no trace left to show that the system had undergone thermodynamic change.
4