Page 30 - FINAL DBS10012_HEAT TEMPERATURE 20102022 (2) (1)
P. 30
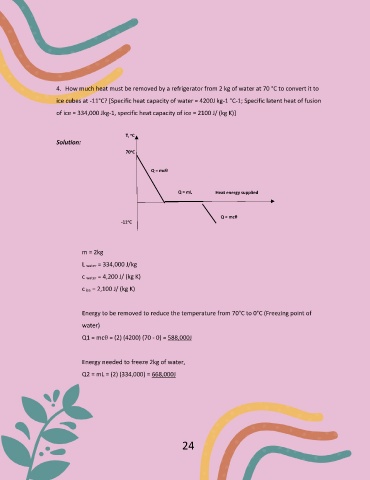
4. How much heat must be removed by a refrigerator from 2 kg of water at 70 °C to convert it to
ice cubes at -11°C? [Specific heat capacity of water = 4200J kg-1 °C-1; Specific latent heat of fusion
of ice = 334,000 Jkg-1, specific heat capacity of ice = 2100 J/ (kg K)]
T, C
o
Solution:
70 C
o
Q = mcθ
Q = mL Heat energy supplied
Q = mcθ
o
-11 C
m = 2kg
L water = 334,000 J/kg
c water = 4,200 J/ (kg K)
c ice = 2,100 J/ (kg K)
Energy to be removed to reduce the temperature from 70°C to 0°C (Freezing point of
water)
Q1 = mcθ = (2) (4200) (70 - 0) = 588,000J
Energy needed to freeze 2kg of water,
Q2 = mL = (2) (334,000) = 668,000J
24