Page 93 - MATERIAL SCIENCE AND ENGINEERING PART 1
P. 93
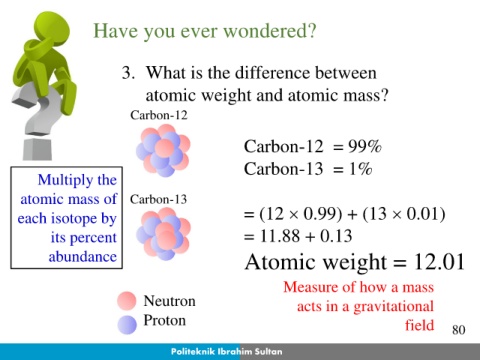
Have you ever wondered?
3. What is the difference between
atomic weight and atomic mass?
Carbon-12
Carbon-12 = 99%
Carbon-13 = 1%
Multiply the
atomic mass of Carbon-13
each isotope by = (12 0.99) + (13 0.01)
its percent = 11.88 + 0.13
abundance Atomic weight = 12.01
Electron Measure of how a mass
Neutron acts in a gravitational
Proton field 80
Politeknik Ibrahim Sultan